Statistical analysis of σ-holes: a novel complementary view on halogen bonding
Abstract
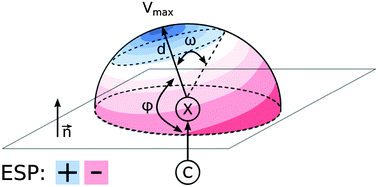
To contribute to the understanding of noncovalent binding of halogenated molecules with biological activity, electrostatic potential (ESP) maps of more than 2500 compounds were thoroughly analysed. A peculiar region of positive ESP, called the σ-hole, is a concept of central importance for halogen bonding. We aim to simplify the view on σ-holes and to provide general trends in organic drug-like molecules. The results are in fair agreement with crystallographic surveys of small molecules as well as biomolecular complexes and may help to improve the intuition of chemists when dealing with halogenated compounds.

 Please wait while we load your content...
Please wait while we load your content...