Assessing the hydration free energy of a homologous series of polyols with classical and quantum mechanical solvation models
Abstract
Molecular dynamics (MD) simulations associated with the thermodynamic integration (TI) scheme and the polarizable continuum model (PCM) in combination with the SMD solvation model were used to study the hydration free energy of the homologous series of polyols, CnHn+2(OH)n (1 ≤ n ≤ 7). Both solvation models predict a nonlinear behavior for the hydration free energy with the increase of the number of hydroxyl groups. This study also indicates that there is a sizable solute polarization in aqueous solution and that the inclusion of the polarization effect is important for a reliable description of the free energy differences considered here.
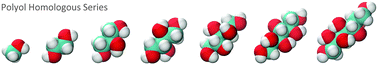

 Please wait while we load your content...
Please wait while we load your content...