Solvent induced conformer specific photochemistry of guaiacol†
Abstract
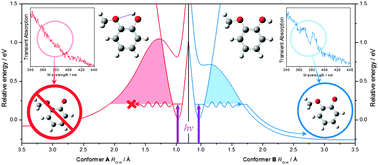
Using a combination of ultrafast solution- and gas-phase spectroscopies, together with high-level theory calculations, we demonstrate that we are able to track conformer-specific photodissociation dynamics in solution through solvent choice. We reveal this phenomenon in guaiacol (2-methoxyphenol), a key subunit of the natural biopolymer lignin. In cyclohexane, the first electronically excited 1ππ* (S1) state in guaiacol relaxes with a time-constant of τ = 4.5 ± 0.2 ns, mediated through intersystem crossing to lower lying triplet (Tn) states and internal conversion and fluorescence back to the ground state (S0). In contrast, in methanol, a further relaxation channel is also present; the S1 state relaxes with a time-constant of τ = 2.9 ± 0.1 ns, which is now additionally mediated through coupling onto a dissociative 1πσ* (S2) state and subsequent O–H bond fission, evidenced through the appearance of a spectral signature for the guaiacoxyl radical after ∼250 ps. With the aid of complementary calculations, we attribute this to the now absent intramolecular H-bond between OH and OMe moieties, which now favours intermolecular H-bonding to methanol, lowering the barrier to O–H dissociation and facilitating H-atom loss via tunnelling.

 Please wait while we load your content...
Please wait while we load your content...