Strongly bound noncovalent (SO3)n:H2CO complexes (n = 1, 2)†
Abstract
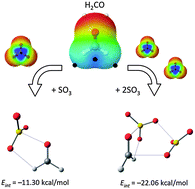
The potential energy surfaces (PES) for the SO3:H2CO and (SO3)2:H2CO complexes were thoroughly examined at the MP2/aug-cc-pVDZ computational level. Heterodimers and trimers are held together primarily by S⋯O chalcogen bonds, supplemented by weaker CH⋯O and/or O⋯C bonds. The nature of the interactions is probed by a variety of means, including electrostatic potentials, AIM, NBO, energy decomposition, and electron density redistribution maps. The most stable dimer is strongly bound, with an interaction energy exceeding 10 kcal mol−1. Trimers adopt the geometry of the most stable dimer, with an added SO3 molecule situated so as to interact with both of the original molecules. The trimers are strongly bound, with total interaction energies of more than 20 kcal mol−1. Most such trimers show positive cooperativity, with shorter S⋯O distances, and three-body interaction energies of nearly 3 kcal mol−1.

 Please wait while we load your content...
Please wait while we load your content...