Structures, mechanical properties, equations of state, and electronic properties of β-HMX under hydrostatic pressures: a DFT-D2 study
Abstract
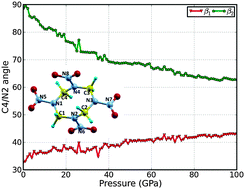
We report the hydrostatic compression studies of the β-polymorph of a cyclotetramethylene tetranitramine (HMX) energetic molecular crystal using DFT-D2, a first-principles calculation based on density functional theory (DFT) with van der Waals (vdW) corrections. The molecular structure, mechanical properties, electronic properties, and equations of state of β-HMX are investigated. For the first time, we predict the elastic constants of β-HMX using DFT-D2 studies. The equations of state under hydrostatic compression are studied for pressures up to 100 GPa. We found that the N–N bonds along the minor axis are responsible for the sensitivity of β-HMX. The analysis of the charge distribution shows that the electronic charge is transferred from hydrogen atoms to nitro groups with the amount of 0.131 and 0.064e for the nitro groups along the minor axis and major axis, respectively, when pressure changes from 0 GPa to 100 GPa. The electronic energy band gap changes from direct at a pressure of 0 GPa to indirect at a pressure of 50 GPa and higher. The band gap decreases with respect to an increase in pressure, implying that the impact sensitivity increases with compression. Our study suggests that the van der Waals interactions are critically important in modeling the mechanical properties of this molecular crystal.

 Please wait while we load your content...
Please wait while we load your content...