Iron cluster–CO bond energies from the kinetic energy dependence of the Fen+ (n = 4–17) + CO association reactions†
Abstract
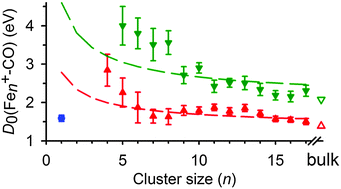
The energy dependences of size-selected Fen+ cluster ions (n = 1–17) reacting with CO at low energies are studied using a guided ion beam tandem mass spectrometer equipped with a laser vaporization source. Below about 1 eV, all clusters with n ≥ 4 form the FenCO+ association complex. The probability of this reaction increases with cluster size until the absolute cross sections equal the collision limit for n > 10, with those for n = 12 and 14 exceeding the collision limit. As the cluster size increases, a second pathway becomes prominent at higher energies. The kinetic energy dependence and absolute magnitudes of these cross sections are analyzed to extract cluster–CO binding energies, which are found to progressively decrease with increasing cluster size, consistent with a simple Coulomb model. For the largest clusters, these binding energies approach that of an extended surface at shallow hollow sites, whereas the higher energy feature correlates with binding energies for dissociatively chemisorbed C and O on an iron surface.
- This article is part of the themed collection: Size Selected Clusters and Particles: From Physical Chemistry to Catalysis

 Please wait while we load your content...
Please wait while we load your content...