An experimental and theoretical investigation of the N(4S) + C2(1Σg+) reaction at low temperature
Abstract
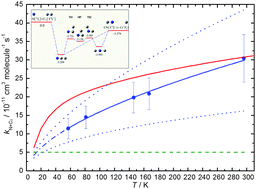
Rate constants for the N(4S) + C2(1Σg+) reaction have been measured in a continuous supersonic flow reactor over the range 57 K ≤ T ≤ 296 K by the relative rate technique employing the N(4S) + OH(X2Π) → H(2S) + NO(X2Π) reaction as a reference. Excess concentrations of atomic nitrogen were produced by the microwave discharge method and C2 and OH radicals were created by the in situ pulsed laser photolysis of precursor molecules C2Br4 and H2O2 respectively. In parallel, quantum dynamics calculations were performed based on an accurate global potential energy surfaces for the three lowest lying quartet states of the C2N molecule. The 14A′′ potential energy surface is barrierless, having two deep potential wells corresponding to the NCC and CNC intermediates. Both the experimental and theoretical work show that the rate constant decreases to low temperature, although the experimentally measured values fall more rapidly than the theoretical ones except at the lowest temperatures. Astrochemical simulations indicate that this reaction could be the dominant source of CN in dense interstellar clouds.

 Please wait while we load your content...
Please wait while we load your content...