A quantum chemical topological analysis of the C–C bond formation in organic reactions involving cationic species
Abstract
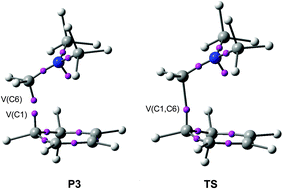
ELF topological analysis of the ionic Diels–Alder (I-DA) reaction between the N,N-dimethyliminium cation and cyclopentadiene (Cp) has been performed in order to characterise the C–C single bond formation. The C–C bond formation begins in the short range of 2.00–1.96 Å via a C-to-C pseudoradical coupling between the most electrophilic center of the iminium cation and one of the two most nucleophilic centers of Cp. The electron density of the pseudoradical center generated at the most electrophilic carbon of the iminium cation comes mainly from the global charge transfer which takes place along the reaction. Analysis of the global reactivity indices indicates that the very high electrophilic character of the iminium cation is responsible for the negative activation energy found in the gas phase. On the other hand, the analysis of the radical Pok Parr functions of the iminium cation, and the nucleophilic Pk− Parr functions of Cp makes the characterisation of the most favourable two-center interaction along the formation of the C–C single bond possible.

 Please wait while we load your content...
Please wait while we load your content...