Electrochemical CO2 reduction on Cu2O-derived copper nanoparticles: controlling the catalytic selectivity of hydrocarbons†
Abstract
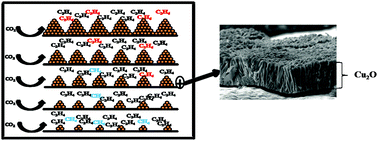
The catalytic activity and hydrocarbon selectivity in electrochemical carbon dioxide (CO2) reduction on cuprous oxide (Cu2O) derived copper nanoparticles is discussed. Cuprous oxide films with [100], [110] and [111] orientation and variable thickness were electrodeposited by reduction of copper(II) lactate on commercially available copper plates. After initiation of the electrochemical CO2 reduction by these oxide structures, the selectivity of the process was found to largely depend on the parent Cu2O film thickness, rather than on the initial crystal orientation. Starting with thin Cu2O films, besides CO and hydrogen, selective formation of ethylene is observed with very high ethylene-to-methane ratios (∼8 to 12). In addition to these products, thicker Cu2O films yield a remarkably large amount of ethane. Long term Faradaic efficiency analysis of hydrocarbons shows no sign of deactivation of the electrodes after 5 hours of continuous experiment. Online mass spectroscopy studies combined with X-ray diffraction data suggest the reduction of the Cu2O films in the presence of CO2, generating a nanoparticulate Cu morphology, prior to the production of hydrogen, CO, and hydrocarbons. Optimizing coverage, number density and size of the copper nanoparticles, as well as local surface pH, may allow highly selective formation of the industrially important product ethylene.

 Please wait while we load your content...
Please wait while we load your content...