Analysis of hydrogen bond energies and hydrogen bonded networks in water clusters (H2O)20 and (H2O)25 using the charge-transfer and dispersion terms
Abstract
The hydrogen bonds and their networks in the water clusters (H2O)20 and (H2O)25 are characterized using the charge-transfer 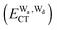 and dispersion
and dispersion 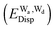 terms for every pair of water molecules (Wa, Wd) in the clusters. The terms are evaluated by the perturbation theory based on the ab initio locally projected molecular orbitals (LPMO PT) developed by the present author. The relative binding energies among the isomers evaluated by the LPMO PT agree with those of the high level ab initio wave function based theories. A strong correlation between
terms for every pair of water molecules (Wa, Wd) in the clusters. The terms are evaluated by the perturbation theory based on the ab initio locally projected molecular orbitals (LPMO PT) developed by the present author. The relative binding energies among the isomers evaluated by the LPMO PT agree with those of the high level ab initio wave function based theories. A strong correlation between 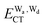 and
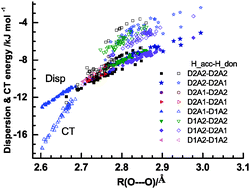
and 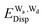 for the hydrogen bonded pairs is found. The pair-wise interaction energies are characterized by the types of hydrogen-donor (Wd) and hydrogen-acceptor (Wa) water molecules. The strongest pair is that of the D2A1 water molecule as a hydrogen-acceptor and the D1A2 water molecule as a hydrogen-donor, where the DnAm water molecule implies that the water molecule has n hydrogen bonding O–H and m accepting H⋯O. The intra-molecular deformation as well as the O⋯O distance is also dependent on the types of hydrogen bonded pairs. The ring structures in the cluster are classified by the pattern of alignment of the hydrogen bonds. The lengthening of the hydrogen-bonding OH of Wd is strongly correlated with the charge-transfer
for the hydrogen bonded pairs is found. The pair-wise interaction energies are characterized by the types of hydrogen-donor (Wd) and hydrogen-acceptor (Wa) water molecules. The strongest pair is that of the D2A1 water molecule as a hydrogen-acceptor and the D1A2 water molecule as a hydrogen-donor, where the DnAm water molecule implies that the water molecule has n hydrogen bonding O–H and m accepting H⋯O. The intra-molecular deformation as well as the O⋯O distance is also dependent on the types of hydrogen bonded pairs. The ring structures in the cluster are classified by the pattern of alignment of the hydrogen bonds. The lengthening of the hydrogen-bonding OH of Wd is strongly correlated with the charge-transfer 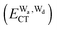 energy.
energy.

 Please wait while we load your content...
Please wait while we load your content...