Chemical stability and degradation mechanisms of triangular Ag, Ag@Au, and Au nanoprisms†
Abstract
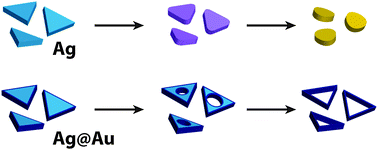
Anisotropic metal nanoparticles have found use in a variety of plasmonic applications because of the large near-field enhancements associated with them; however, the very features that give rise to these enhancements (e.g., sharply curved edges and tips) often have high surface energies and are easily degraded. This paper describes the stability and degradation mechanisms of triangular silver, gold-coated silver, and gold nanoprisms upon exposure to a wide variety of adverse conditions, including halide ions, thiols, amines and elevated temperatures. The silver nanoprisms were immediately and irreversibly degraded under all of the conditions studied. In contrast, the core–shell Ag@Au nanoprisms were less susceptible to etching by chlorides and bromides, but were rapidly degraded by iodides, amines and thiols by a different degradation pathway. Only the pure gold nanoprisms were stable to all of the conditions tested. These results have important implications for the suitability of triangular nanoprisms in many applications; this is particularly true in biological or environmental fields, where the nanoparticles would inevitably be exposed to a wide variety of chemical stimuli.

 Please wait while we load your content...
Please wait while we load your content...