Theoretical predictions of isotope effects versus their experimental values for an example of uncatalyzed hydrolysis of atrazine†
Abstract
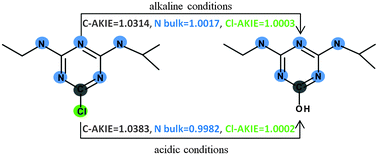
Kinetic isotope effects are one of the most powerful experimental techniques for establishing the nature of a chemical process. However their interpretation very often seeks support from electronic structure calculations in order to get detailed information regarding the transition state which is not experimentally available. For an example of atrazine hydrolysis we have shown how the match between experimentally and theoretically determined magnitudes of carbon, nitrogen and chlorine kinetic isotope effects can be used to discuss the mechanism under different reaction conditions. Two different density functionals combined with the explicit presence of solvent molecules and a continuum solvation model revealed that although the reaction proceeds via the same concerted mechanism regardless of the reaction conditions the transition state structure for an acid and base-catalyzed pathway is different.

 Please wait while we load your content...
Please wait while we load your content...