The single GUV method for revealing the functions of antimicrobial, pore-forming toxin, and cell-penetrating peptides or proteins
Abstract
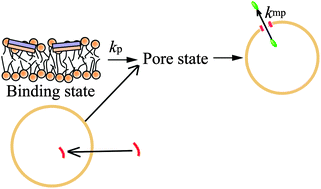
We recently developed the single giant unilamellar vesicle (GUV) method for investigating the functions and dynamics of biomembranes. The single GUV method can provide detailed information on the elementary processes of physiological phenomena in biomembranes, such as their rate constants. Here we describe the process of pore formation induced by the antimicrobial peptide (AMP), magainin 2, and the pore-forming toxin (PFT), lysenin, as revealed by the single GUV method. We obtained the rate constants of several elementary steps, such as peptide/protein-induced pore formation in lipid membranes and the membrane permeation of fluorescent probes through the pores. Information on the entry of the cell-penetrating peptide (CPP), transportan 10 (TP10), into a single GUV and its induced pore formation in lipid membranes was also obtained. We compare the single GUV method with other methods for investigating the interaction of peptides/proteins with lipid membranes (i.e., the large unilamellar vesicle (LUV) suspension method, the GUV suspension method, and single channel recording), and discuss the pros and cons of the single GUV method. On the basis of these data, we discuss the advantages of the single GUV method.

 Please wait while we load your content...
Please wait while we load your content...