Electrolyte layering at the calcite(104)–water interface indicated by Rb+- and Se(vi) K-edge resonant interface diffraction†
Abstract
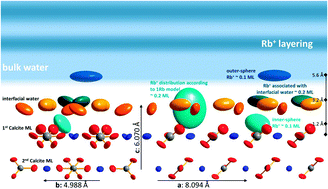
Calcite–water interface reactions are of major importance in various environmental settings as well as in industrial applications. Here we present resonant interface diffraction results on the calcite(104)–aqueous solution interface, measured in solutions containing either 10 mmol L−1 RbCl or 0.5 mmol L−1 Se(VI). Results indicate that Rb+ ions enter the surface adsorbed water layers and adsorb at the calcite(104)–water interface in an inner-sphere fashion. A detailed analysis based on specular and off-specular resonant interface diffraction data reveals three distinct Rb+ adsorption species: one 1.2 Å above the surface, the second associated with surface adsorbed water molecules 3.2 Å above the surface, and the third adsorbed in an outer-sphere fashion 5.6 Å above the surface. A peak in resonant amplitude between L = 1.5 and L = 3.0 is interpreted as signal from a layered electrolyte structure. The presence of a layered electrolyte structure seems to be confirmed by data measured in the presence of Se(VI).

 Please wait while we load your content...
Please wait while we load your content...