The weak, fluctuating, dipole moment of membrane-bound hydrogenase from Aquifex aeolicus accounts for its adaptability to charged electrodes†
Abstract
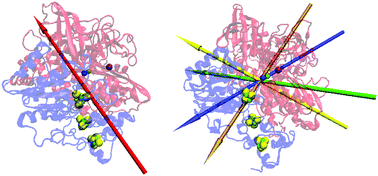
[NiFe] hydrogenases from Aquifex aeolicus (AaHase) and Desulfovibrio fructosovorans (DfHase) have been mainly studied to characterize physiological electron transfer processes, or to develop biotechnological devices such as biofuel cells. In this context, it remains difficult to control the orientation of AaHases on electrodes to achieve a fast interfacial electron transfer. Here, we study the electrostatic properties of these two proteins based on microsecond-long molecular dynamics simulations that we compare to voltammetry experiments. Our calculations show weak values and large fluctuations of the dipole direction in AaHase compared to DfHase, enabling the AaHase to absorb on both negatively and positively charged electrodes, with an orientation distribution that induces a spread in electron transfer rates. Moreover, we discuss the role of the transmembrane helix of AaHase and show that it does not substantially impact the general features of the dipole moment.

 Please wait while we load your content...
Please wait while we load your content...