The influence of a fibrous carbon envelope on the formation of CoFe nanoparticles for durable electrocatalytic oxygen evolution†
Abstract
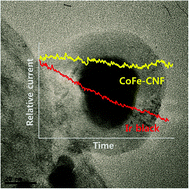
Co oxides are known to be active and stable alternative anode electrocatalysts possessing the potential to replace the best performing but most expensive Ir and Ru oxides in alkaline water electrolysis. Of late, Co oxides loaded on various carbon supports have been reported as a way to outperform Ir or Ru catalysts by improving the utilization efficiency. In this study, we introduce Co and Fe nanoparticles embedded carbon nanofibers (CoFe-CNFs), fabricated through electrospinning and pyrolysis of a polymer mixed with Co and Fe precursors. This method is a facile route for simultaneously making Co and Fe nanoparticles as well as the stable accommodation of the CoFe nanoparticles in the carbon support. We demonstrate the potential of the CoFe-CNFs as active and stable electrocatalysts for the oxygen evolution reaction (OER) in alkaline media. We conducted detailed physico-chemical characterizations to elucidate the effect of the CNFs on the OER activity and stability of the CoFe-CNFs. It is suggested that the CNFs are a medium in which OER-active CoFe alloy nanoparticles are formed homogeneously, and that carbon layers surrounding the nanoparticles are beneficial to the stability of the CoFe-CNFs in the OER.
- This article is part of the themed collection: Electrocatalysis - Fundamental Insights for Sustainable Energy

 Please wait while we load your content...
Please wait while we load your content...