Density functional theory study of carbon dioxide electrochemical reduction on the Fe(100) surface
Abstract
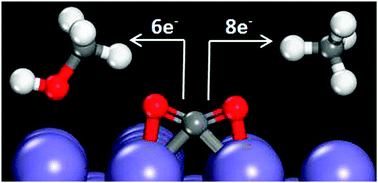
Carbon dioxide electroreduction offers the possibility of producing hydrocarbon fuels using energy from renewable sources. Herein, we use density functional theory to analyze the feasibility of CO2 electroreduction on a Fe(100) surface. Experimentally, iron is nonselective for hydrocarbon formation. A simplistic analysis of low-coverage reaction intermediate energies for the paths to produce CH4 and CH3OH from CO2 suggests Fe(100) could be more active than Cu(111), currently the only metallic catalyst to show selectivity towards hydrocarbon formation. We consider a series of impediments to CO2 electroreduction on Fe(100) including O*/OH* (* denotes surface bound species) blockage of active surface sites; competitive adsorption effects of H*, CO* and C*; and iron carbide formation. Our results indicate that under CO2 electroreduction conditions, Fe(100) is predicted to be covered in C* or CO* species, blocking any C–H bond formation. Further, bulk Fe is predicted to be unstable relative to FeCx formation at potentials relevant to CO2 electroreduction conditions.
- This article is part of the themed collection: Electrocatalysis - Fundamental Insights for Sustainable Energy

 Please wait while we load your content...
Please wait while we load your content...