Following ORR intermediates adsorbed on a Pt cathode catalyst during break-in of a PEM fuel cell by in operando X-ray absorption spectroscopy
Abstract
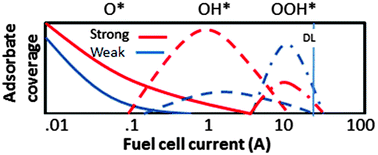
In operando X-ray absorption spectroscopy data using the Δμ X-ray Absorption Near Edge Spectroscopy (XANES) analysis procedure is used to follow the ORR intermediate adsorbate coverage on a working catalyst in a PEMFC during initial activation and break-in. The adsorbate coverage and log i (Tafel) curves reveal a strong correlation, i.e., an increase in adsorbate intermediate coverage poisons Pt sites thereby decreasing the current. A decrease in Pt–O bond strength commensurate with decrease in potential causes a sequence of different dominant adsorbate volcano curves to exist, namely first O, then OH, and then OOH exactly as predicted by the different ORR kinetics mechanisms. During break-in, the incipient O coverage coming from exposure to air during storage and MEA preparation is rather quickly removed, compared to the slower and more subtle nanoparticle morphological changes, such as the rounding of the Pt nanoparticle edges/corners and smoothing of the planar surfaces, driven by the nanoparticle's tendency to lower its surface energy. These morphological changes increase the Pt–Pt average coordination number, decrease the average Pt–O bond strength, and thereby decrease the coverage of ORR intermediates, allowing increase in the current.
- This article is part of the themed collection: Electrocatalysis - Fundamental Insights for Sustainable Energy

 Please wait while we load your content...
Please wait while we load your content...