Application of a planar superatom model on [Hg5(C(CF3)2)]. Bonding and magnetic response considerations into a five-fold d10–d10 metal cycle†
Abstract
Application of the planar superatom model to describe the electronic structure, and to gain insights into the stabilization of metal macrocycles supporting closed-shell d10–d10 interactions, is studied through analysis of the membered ring composed by Hg(II) atoms, namely, [Hg(C(CF3)2)]5. Its electronic structure resembles the level sequence given for a planar jellium model, revealing an electronic configuration given by 1s2 1p4x,y 1d4xy,x2−y2. The analysis of the population of each level of the Hg5core, denotes a slight net bonding into the [Hg(C(CF3)2)]5 ring. However, stabilization is mainly supported by the balance given by a similar population of the jellium levels, which is suggested to be a different scheme for stabilizing d10 macrocyclic clusters with metallophilic interactions, in the category of long d10–d10 contacts. The extension of the planar jellium model to the relativistic case, including spin–orbit coupling considering the D5h* point group, denotes the consequent splitting for levels with 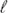 ≠ 0, namely, 1px,y and 1dxy,x2−y2. In this framework, the electronic configuration is given by 1s1/22 1p3/22 1p1/22 1d5/22 1d3/22, which contribute to the analysis of the electronic structure of planar clusters in terms of spin–orbit coupling, involving molecular spinors (j =
≠ 0, namely, 1px,y and 1dxy,x2−y2. In this framework, the electronic configuration is given by 1s1/22 1p3/22 1p1/22 1d5/22 1d3/22, which contribute to the analysis of the electronic structure of planar clusters in terms of spin–orbit coupling, involving molecular spinors (j = 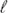 ± s) instead of molecular orbitals (pure
± s) instead of molecular orbitals (pure 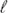 ).
).
![Graphical abstract: Application of a planar superatom model on [Hg5(C(CF3)2)]. Bonding and magnetic response considerations into a five-fold d10–d10 metal cycle](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C3CP55482A&imageInfo.ImageIdentifier.Year=2014)

 Please wait while we load your content...
Please wait while we load your content...