A water-soluble tin(iv) porphyrin as a bioinspired photosensitiser for light-driven proton-reduction†
Abstract
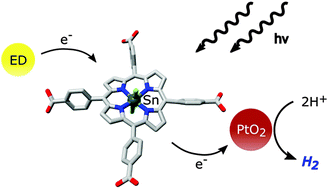
The water-soluble tin(IV) porphyrin dichlorido-5,10,15,20-tetrakis(p-carboxyphenyl)-porphyrinato-tin(IV) (SnTPPC, 1) was synthesised as a mimic of biological chlorophyll photosensitisers. In natural photosynthesis, chlorophyll pigments start the multi-electron transfer processes resulting in water-oxidation and NADP+-reduction. The photochemical properties of compound 1 were characterised by measuring absorption and fluorescence spectra. Electrochemical measurements in water revealed well-suited redox potentials of 1 for both proton-reduction to H2 as well as water-oxidation to O2. The tin(IV) porphyrin was then used as a photosensitiser in model systems for light-induced proton-reduction in aqueous solution, where an optimization of the experimental conditions was carried out to achieve reaction rates comparable to those found for [Ru(bipy)3]2+, a standard dye in artificial photosynthesis. By employing UV/Vis-spectroelectrochemistry, we found that the porphyrin ligand of 1 is redox non-innocent in water. A complex set of reduction reactions of the porphyrin macrocycle occurs during photocatalytic experiments involving the ligand's chlorin form as a key intermediate. On the basis of these results, a potential reaction sequence for light-driven H2-formation is formulated, where the reductive quenching of 1 forms the initial reaction step and reduced forms of 1 serve as hydride transfer agents to the H2 evolution catalyst. The spectroscopic, electrochemical and catalytic properties of SnTPPC make this compound class an attractive, affordable and easily accessible choice for photosensitisers in artificial photosynthetic systems. Finally, the detected complicated redox reactions of the porphyrin ring in water offer a possible explanation of why the chlorophylls of P680 or P700 are carefully wrapped in a water-free part of the PSII and PSI proteins.
- This article is part of the themed collection: Photosynthesis: From Natural to Artificial

 Please wait while we load your content...
Please wait while we load your content...