Diffuse transition state structure for the unfolding of a leucine-rich repeat protein
Abstract
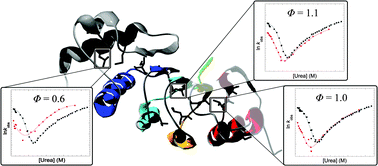
Tandem-repeat proteins, such as leucine-rich repeats, comprise arrays of small structural motifs that pack in a linear fashion to produce elongated architectures. They lack contacts between residues that are distant in primary sequence, a feature that distinguishes them from the complex topologies of globular proteins. Here we have investigated the unfolding pathway of the leucine-rich repeat domain of the mRNA export protein TAP (TAPLRR) using Φ-value analysis. Whereas most of the tandem-repeat proteins studied to date have been found to unfold via a polarised mechanism in which only a small, localised number of repeats are structured in the transition state, the unfolding mechanism of TAPLRR is more diffuse in nature. In the transition state for unfolding of TAPLRR, three of the four LRRs are highly structured and non-native interactions are formed within the N-terminal α-helical cap and the first LRR. Thus, the α-helical cap plays an important role in which non-native interactions are required to provide a scaffold for the LRRs to pack against in the folding reaction.
- This article is part of the themed collection: The free energy landscape: from folding to cellular function

 Please wait while we load your content...
Please wait while we load your content...