A combined experimental and theoretical study of reactions between the hydroxyl radical and oxygenated hydrocarbons relevant to astrochemical environments
Abstract
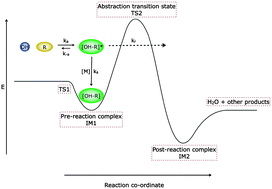
The kinetics of the reactions of the hydroxyl radical (OH) with acetone and dimethyl ether (DME) have been studied between 63–148 K and at a range of pressures using laser-flash photolysis coupled with laser induced fluorescence detection of OH in a pulsed Laval nozzle apparatus. For acetone, a large negative temperature dependence was observed, with the rate coefficient increasing from k1 = (1.6 ± 0.8) × 10−12 cm3 molecule−1 s−1 at 148 K to (1.0 ± 0.1) × 10−10 cm3 molecule−1 s−1 at 79 K, and also increasing with pressure. For DME, a similar behaviour was found, with the rate coefficient increasing from k2 = (3.1 ± 0.5) × 10−12 cm3 molecule−1 s−1 at 138 K to (1.7 ± 0.1) × 10−11 cm3 molecule−1 s−1 at 63 K, and also increasing with pressure. The temperature and pressure dependence of the experimental rate coefficients are rationalised for both reactions by the formation and subsequent stabilisation of a hydrogen bonded complex, with a non-zero rate coefficient extrapolated to zero pressure supportive of quantum mechanical tunnelling on the timescale of the experiments leading to products. In the case of DME, experiments performed in the presence of O2 provide additional evidence that the yield of the CH3OCH2 abstraction product, which can recycle OH in the presence of O2, is ≥50%. The experimental data are modelled using the MESMER (Master Equation Solver for Multi Energy Well Reactions) code which includes a treatment of quantum mechanical tunnelling, and uses energies and structures of transition states and complexes calculated by ab initio methods. Good agreement is seen between experiment and theory, with MESMER being able to reproduce for both reactions the temperature behaviour between ∼70–800 K and the pressure dependence observed at ∼80 K. At the limit of zero pressure, the model predicts a rate coefficient of ∼10−11 cm3 molecule−1 s−1 for the reaction of OH with acetone at 20 K, providing evidence that the reaction can proceed quickly in those regions of space where both species have been observed. The results and modelling build considerably on our previous experimental study performed under a much more limited range of conditions (Shannon et al., Phys. Chem. Chem. Phys., 2010, 12, 13511–13514).
- This article is part of the themed collection: Astrochemistry

 Please wait while we load your content...
Please wait while we load your content...