Predicted disorder-to-order transition mutations in IκBα disrupt function†
Abstract
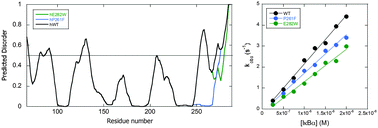
IκBα inhibits the transcription factor, NFκB, by forming a very tightly bound complex in which the ankyrin repeat domain (ARD) of IκBα interacts primarily with the dimerization domain of NFκB. The first four ankyrin repeats (ARs) of the IκBα ARD are well-folded, but the AR5–6 region is intrinsically disordered according to amide H/D exchange and protein folding/unfolding experiments. We previously showed that mutations towards the consensus sequence for stable ankyrin repeats resulted in a “prefolded” mutant. To investigate whether the consensus mutations were solely able to order the AR5–6 region, we used a predictor of protein disordered regions PONDR VL-XT to select mutations that would alter the intrinsic disorder towards a more ordered structure (D → O mutants). The algorithm predicted two mutations, E282W and P261F, neither of which correspond to the consensus sequence for ankyrin repeats. Amide exchange and CD were used to assess ordering. Although only the E282W was predicted to be more ordered by CD and amide exchange, stopped-flow fluorescence studies showed that both of the D → O mutants were less efficient at dissociating NFκB from DNA.
- This article is part of the themed collection: The free energy landscape: from folding to cellular function

 Please wait while we load your content...
Please wait while we load your content...