Atomic oxygen diffusion on and desorption from amorphous silicate surfaces
Abstract
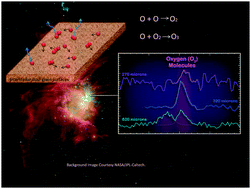
Surface reactions involving atomic oxygen have attracted much attention in astrophysics and astrochemistry, but two of the most fundamental surface processes, desorption and diffusion, are not well understood. We studied diffusion and desorption of atomic oxygen on or from amorphous silicate surfaces under simulated interstellar conditions using a radio-frequency dissociated oxygen beam. Temperature programmed desorption (TPD) experiments were performed to study the formation of ozone from reaction of atomic and molecular oxygen deposited on the surface of a silicate. It is found that atomic oxygen begins to diffuse significantly between 40 K and 50 K. A rate equation model was used to study the surface kinetics involved in ozone formation experiments. The value of atomic oxygen desorption energy has been determined to be 152 ± 20 meV (1764 ± 232 K). The newly found atomic oxygen desorption energy, which is much higher than the well-accepted value, might explain the discrepancy in abundance of molecular oxygen in space between observations and chemical models.
- This article is part of the themed collection: Astrochemistry

 Please wait while we load your content...
Please wait while we load your content...