A kinetic study of domain swapping of Protein L†
Abstract
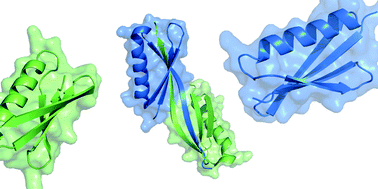
Domain swapping of the B1 domain of Protein L isolated from Peptostreptococcus magnus can be induced by rational mutation. We show that the monomeric and the domain swapped dimeric forms of the G55A mutant of Protein L are directly observable by solution NMR spectroscopy under equilibrium conditions. The kinetics of the domain swapping process can be characterized by real-time NMR spectroscopic techniques, and the free energy landscape for domain swapping of Protein L can be probed by variation of denaturant concentration. Our data suggest that domain swapping of Protein L proceeds through a compact transition state, with an accessible surface area that is similar in size to the transition state for folding and unfolding. It is thus conceivable that domain swapping and folding of Protein L are mechanistically related at the level of their rate-limiting step(s), which might represent a branching point along the folding pathway.
- This article is part of the themed collection: The free energy landscape: from folding to cellular function

 Please wait while we load your content...
Please wait while we load your content...