Interactions of gaseous HNO3 and water with individual and mixed alkyl self-assembled monolayers at room temperature
Abstract
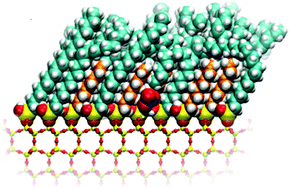
The major removal processes for gaseous nitric acid (HNO3) in the atmosphere are dry and wet deposition onto various surfaces. The surface in the boundary layer is often covered with organic films, but the interaction of gaseous HNO3 with them is not well understood. To better understand the factors controlling the uptake of gaseous nitric acid and its dissociation in organic films, studies were carried out using single component and mixtures of C8 and C18 alkyl self-assembled monolayers (SAMs) attached to a germanium (Ge) attenuated total reflectance (ATR) crystal upon which a thin layer of SiOx had been deposited. For comparison, diffuse reflectance infrared Fourier transform spectrometry (DRIFTS) studies were also carried out using a C18 SAM attached to the native oxide layer on the surface of silicon powder. These studies show that the alkyl chain length and order/disorder of the SAMs does not significantly affect the uptake or dissociation/recombination of molecular HNO3. Thus, independent of the nature of the SAM, molecular HNO3 is observed up to 70–90% relative humidity. After dissociation, molecular HNO3 is regenerated on all SAM surfaces when water is removed. Results of molecular dynamics simulations are consistent with experiments and show that defects and pores on the surfaces control the uptake, dissociation and recombination of molecular HNO3. Organic films on surfaces in the boundary layer will certainly be more irregular and less ordered than SAMs studied here, therefore undissociated HNO3 may be present on surfaces in the boundary layer to a greater extent than previously thought. The combination of this observation with the results of recent studies showing enhanced photolysis of nitric acid on surfaces suggests that renoxification of deposited nitric acid may need to be taken into account in atmospheric models.

 Please wait while we load your content...
Please wait while we load your content...