Effect of the structural difference between Bax-α5 and Bcl-xL-α5 on their interactions with lipid bilayers
Abstract
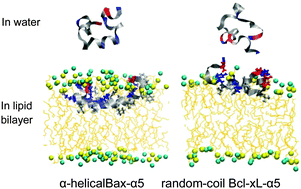
Bax-α5 and Bcl-xL-α5, which are shorter versions of apoptosis-regulating proteins Bax and Bcl-xL, were simulated with lipid bilayers composed of pure dioleoylglycerophosphocholine (DOPC) lipids or a mixture of DOPCs and cholesterols. Starting with the initial peptide position near the bilayer surface, both Bax-α5 and Bcl-xL-α5 bind to the bilayer because of their charge interactions with lipid head groups. After binding to the bilayer surface, Bax-α5 inserts into the pure DOPC bilayer, but not into the DOPC–cholesterol bilayer, showing the effect of cholesterols on the peptide–bilayer interaction. Despite the similar peptide structure, Bcl-xL-α5 does not insert into the bilayer, in contrast to the interaction of Bax-α5 with the bilayer. Bcl-xL-α5 predominantly has the random-coil structure in both aqueous and membrane environments, while Bax-α5 shows a higher extent of α-helical structure in the bilayer than in water, in quantitative agreement with experiment. In particular, although Bax-α5 and Bcl-xL-α5 have the same extent of the electrostatic interaction with lipid head groups, Bax-α5 has stronger hydrophobic interaction with lipid tails than does Bcl-xL-α5. These indicate that Bax-α5 retains α-helical structure, where hydrophobic residues on one side of the α-helix interact with lipid tails and thus can easily attract the peptide into the lipid-tail region, while Bcl-xL-α5 forms a random coil that tends to spread on the bilayer surface and thus has weaker hydrophobic interaction with lipid tails. Our findings help explain the experimental observation that showed that Bax-α5 disorders lipids and induces pore formation, but Bcl-xL-α5 does not.

 Please wait while we load your content...
Please wait while we load your content...