Crystal growth of MOF-5 using secondary building units studied by in situ atomic force microscopy†
Abstract
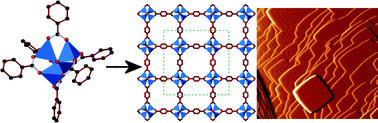
Crystal growth of the metal–organic framework, MOF-5, using basic zinc benzoate, [Zn4O(O2CC6H5)6], was studied in real time using atomic force microscopy. The two-dimensional nuclei involved in layer growth were found to form by a two-step process whereby 1,4-benzenedicarboxylate units first attach to the MOF-5 surface followed by addition of a layer of Zn species and connecting 1,4-benzenedicarboxylate units. No evidence of a growth mechanism involving nucleophilic substitution of a benzoate group from an intact [Zn4O(O2CC6H5)6] molecule by a surface attached 1,4-benzenedicarboxylate unit was found. This indicates that the [Zn4O(O2CC6H5)6] molecules undergo a degree of dissociation before incorporation into the MOF-5 framework. The [Zn4O(O2CC6H5)6]-containing growth solutions were found to influence the relative growth rates along different crystallographic directions and to lead to a faster nucleation rate under certain conditions when compared to growth solutions containing simpler zinc salts. This suggests a degree of remnant association of the zinc species derived from the [Zn4O(O2CC6H5)6] cluster during crystal growth under these conditions.

 Please wait while we load your content...
Please wait while we load your content...