1,2,4,5-Benzenetetrasulfonic acid and 1,4-benzenedisulfonic acid as sulfo analogues of pyromellitic and terephthalic acids for building coordination polymers of manganese†
Abstract
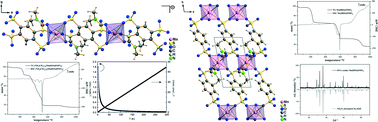
The new polysulfonic acids H4B4S (1,2,4,5-benzenetetrasulfonic acid) and H2BDS (1,4-benzenedisulfonic acid) were used for the preparation of five manganese coordination polymers. The reactions of H4B4S and MnCO3 were performed in dimethylformamide (DMF) or N-methylpyrrolidone (NMP) as solvent in sealed glass ampoules at elevated temperatures. Two benzenetetrasulfonates could be obtained, [NH2(CH3)2]2{Mn(B4S)(DMF)2} (I) (P21/c, Z = 2, a = 942.02(5) pm, b = 1684.86(6) pm, c = 918.45(5) pm, β = 97.746(6)°, R1; wR2 (Io > 2σ(Io)) = 0.0357; 0.0771) and [HNMP]2{Mn(B4S)(NMP)2} (II) (P21/c, Z = 2, a = 931.7(1) pm, b = 1049.2(1) pm, c = 1944.9(1) pm, β = 113.529(3)°, R1; wR2 (Io > 2σ(Io)) = 0.0470; 0.0952). Both compounds exhibit anionic chains according to ∞1{Mn(B4S)2/2(L)2}2− (L = DMF, NMP). The Mn2+ ions are in octahedral coordination of oxygen atoms. The charge of the anionic chains is either compensated by dimethylammonium cations, [NH2(CH3)2+], or by protonated NMP molecules, HNMP+. Solvothermal reactions of H2BDS and MnCO3 in the solvents DMF, NMP and dimethylacetamide (DMA), respectively, yielded the disulfonates Mn(BDS)(DMF)2 (III) (P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , Z = 1, a = 514.3(1) pm, b = 926.2(1) pm, c = 940.2(1) pm, α = 93.552(8)°, β = 99.993(7)°, γ = 99.237(7)°, R1; wR2 (Io > 2σ(Io)) = 0.0290; 0.0637), Mn(BDS)(DMA)2 (IV) (P
, Z = 1, a = 514.3(1) pm, b = 926.2(1) pm, c = 940.2(1) pm, α = 93.552(8)°, β = 99.993(7)°, γ = 99.237(7)°, R1; wR2 (Io > 2σ(Io)) = 0.0290; 0.0637), Mn(BDS)(DMA)2 (IV) (P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , Z = 2, a = 936.00(4) pm, b = 984.94(4) pm, c = 1034.75(5) pm, α = 81.606(2)°, β = 88.941(2)°, γ = 82.364(2)°, R1; wR2 (Io > 2σ(Io)) = 0.0387; 0.1046) and Mn(BDS)(NMP)2 (V) (P
, Z = 2, a = 936.00(4) pm, b = 984.94(4) pm, c = 1034.75(5) pm, α = 81.606(2)°, β = 88.941(2)°, γ = 82.364(2)°, R1; wR2 (Io > 2σ(Io)) = 0.0387; 0.1046) and Mn(BDS)(NMP)2 (V) (P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , Z = 2, a = 962.97(3) pm, b = 976.76(3) pm, c = 1034.23(3) pm, α = 89.371(1)°, β = 78.839(1)°, γ = 87.604(2)°, R1; wR2 (Io > 2σ(Io)) = 0.0243; 0.0713). In the crystal structures of the three compounds, the octahedrally coordinated Mn2+ ions are linked by the disulfonate ions to layers according to ∞2{Mn(BDS)4/4(L)2} (L = DMF, DMA, NMP). The thermal analysis of all compounds shows that they can be desolvated completely and that the remaining solvent-free sulfonates exhibit decomposition temperatures up to 500 °C that is exceptionally high when compared to the respective carboxylates. Temperature-dependent magnetic susceptibility measurements of [NH2(CH3)2]2{Mn(B4S)(DMF)2} show paramagnetic behavior of the Mn2+ ions.
, Z = 2, a = 962.97(3) pm, b = 976.76(3) pm, c = 1034.23(3) pm, α = 89.371(1)°, β = 78.839(1)°, γ = 87.604(2)°, R1; wR2 (Io > 2σ(Io)) = 0.0243; 0.0713). In the crystal structures of the three compounds, the octahedrally coordinated Mn2+ ions are linked by the disulfonate ions to layers according to ∞2{Mn(BDS)4/4(L)2} (L = DMF, DMA, NMP). The thermal analysis of all compounds shows that they can be desolvated completely and that the remaining solvent-free sulfonates exhibit decomposition temperatures up to 500 °C that is exceptionally high when compared to the respective carboxylates. Temperature-dependent magnetic susceptibility measurements of [NH2(CH3)2]2{Mn(B4S)(DMF)2} show paramagnetic behavior of the Mn2+ ions.

 Please wait while we load your content...
Please wait while we load your content...