Molecular structure and polymorphism of a cyclohexanediol: trans-1,4-cyclohexanedimethanol†
Abstract
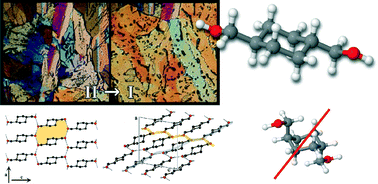
This study aims to investigate the molecular structure and polymorphism of trans-1,4-cyclohexanedimethanol, including the bi-axial/bi-equatorial equilibrium and the nature of the intermolecular H-bond networks in condensed phases created by the hydroxyl group torsions. The full conformational space of the single molecule was explored by MP2 calculations, showing that the optimized bi-equatorial conformers have similar stability and the bi-axial ones have much higher energies. The hydroxymethyl substituents have preference for gauche/anti or gauche+/gauche− conformations. Polymorphic forms were generated by crystallization from solutions and by cooling the melt, which were characterized by a combination of techniques: DSC, PLTM and XRD. Two polymorphs were isolated and their crystal structures were solved by direct methods based on single-crystal X-ray analysis. Both were found to contain two of the most stable conformers found in the computational calculations. The influence of H-bonding in the polymorphic structures was verified by analysis of the structural differences between the geometries present in the polymorphs determined by XRD and their single molecule counterparts resulting from the theoretical calculations. The bi-axial conformations are destabilized over the bi-equatorial ones in isolated and crystalline forms of trans-1,4-cyclohexanedimethanol.

 Please wait while we load your content...
Please wait while we load your content...