Unusual hydrogen bond patterns contributing to supramolecular assembly: conformational study, Hirshfeld surface analysis and density functional calculations of a new steroid derivative†
Abstract
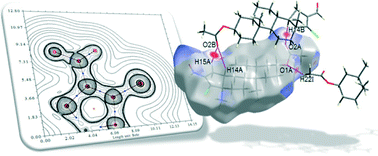
A structural and conformational study of 3β-acetoxy-17-chloro-16-formyl-5α-androstan-16-ene has been carried out by using X-ray analysis and M06-2X density functional calculations. The compound crystallizes with three independent molecules in the asymmetric unit. Natural Bond Order and Atoms in Molecules methods were used for a better understanding of the key factors that determine the stability of this steroidal molecule, particularly the role of C–H⋯Cl intramolecular interactions. A detailed investigation of C–H⋯Cl and C–H⋯O intermolecular interactions, in addition to the most important van der Waals contribution, are presented by means of Hirshfeld surface analysis. The crystal packing exhibits an unusual intra- and intermolecular hydrogen bond pattern, and shows the importance of non-classical interactions in the construction of the supramolecular assembly. Excellent agreement between the theoretical and experimental data is found.

 Please wait while we load your content...
Please wait while we load your content...