Crystallization of tin chloride as a promising pseudocapacitor electrode†
Abstract
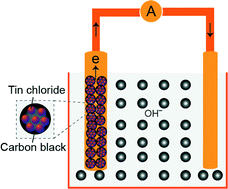
In the present work, we designed an in situ crystallization method for tin chloride salts pseudocapacitors, that is, a SnCl4·5H2O or SnCl2·2H2O electrode in aqueous KOH electrolyte. After undergoing coupled chemical/electrochemical crystallization and Faradaic redox reactions, highly active SnO/Sn colloids were in situ crystallized within the carbon black matrix; such an electrode configuration can result in the fast transfer of ions/electrons and efficiently utilize the active tin cations in the salt electrode, thus high specific capacitance can be obtained. The SnCl4 electrode can deliver ultrahigh specific capacitance of 1592 F g−1 at a current density of 1 A g−1 and a potential range of 0.42 V, which is the highest value among the reported tin-based supercapacitors. Our designed tin chloride electrode/alkaline electrolyte system can offer critical insights into the rational design of the next generation of high-performance supercapacitor devices.

 Please wait while we load your content...
Please wait while we load your content...