Nucleation and crystal growth of amorphous nilutamide – unusual low temperature behavior
Abstract
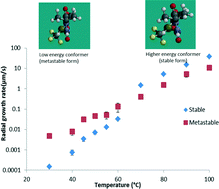
Crystallization from organic undercooled melts is an important topic of investigation, in particular for pharmaceuticals. Nilutamide, a small organic molecule used to treat prostrate cancer, was found to exhibit interesting glass forming and crystallization behavior. Nilutamide readily formed an amorphous solid following melt quenching, despite having a very high crystal growth rate. The good glass forming ability of this compound was attributed to its reluctance to nucleate. Interestingly, when samples were cooled to deep within the glassy region, nucleation to a previously unreported metastable polymorph was observed. Surprisingly, nucleation was found to be more likely when samples were held far below the glass transition temperature (Tg) than when annealed above the Tg. At higher temperatures, the stable polymorph was observed to have the faster growth rate, while at low temperatures, the metastable form grew faster. Conformational analysis suggests that the stable crystal form contains a higher energy conformer which may explain the slow nucleation from the supercooled melt as well as the cross-over in the growth rates of the stable and metastable polymorphs at lower temperatures. This study highlights the complex crystallization behavior of organic supercooled liquids and glasses.
- This article is part of the themed collection: International Year of Crystallography Celebration: North America

 Please wait while we load your content...
Please wait while we load your content...