Molecular symmetry and fluorine-containing supramolecular synthons as structure-differentiating agents in some “bridge-flipped” isomeric bis-benzylideneanilines†
Abstract
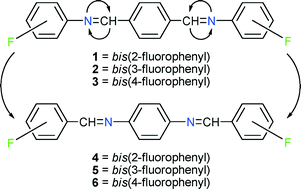
The crystal structures of three pairs of “bridge-flipped isomers” are compared here in the context of whether their similarity in molecular space-filling requirements in combination with the tendency of centrosymmetric molecules to occupy crystallographic inversion centers might lead to their isomorphism. The possibility that similar fluorine-based supramolecular synthons occurring in the crystal structures of both members of a pair might promote their isomorphism is also considered. The compounds are the bis-benzylideneanilines formed by reaction of 2-fluoroaniline, 3-fluoroaniline, and 4-fluoroaniline respectively with terephthaldehyde (1–3) and by reaction of 2-fluorobenzaldehyde, 3-fluorobenzaldehyde, and 4-fluorobenzaldehyde respectively with phenylenediamine (4–6). The crystal structure of 2 is disordered, with the fluorine atom occupying two sites (95 : 5 occupation) related by rotation about the bond between the bridge nitrogen atom and the 3-fluorophenyl group. The crystal structure of 6 (HEWHAU) has been reported by previous workers. The structures of the 1:4, 2:5, and 3:6 pairs are compared to each other and to those of fluorinated simple (one-bridge) benzylideneanilines and fluorinated bis-benzylideneanilines recently described in the literature. No isomorphism is found among the bridge-flipped isomeric pairs, and none is found between positional isomers within the 1–3 or 4–6 series. The supramolecular synthon defined by a 2-F⋯bridge H contact is found in several of the benzylideneaniline crystal structures, but it does not compel isomorphism, nor is it specific to one type (terephthaldehyde-based or phenylenediamine-based) of isomer. Conformational variability and supramolecular synthon variety apparently serve as structure differentiators, not as isomorphism facilitators, among these bis-benzylideneanilines.
- This article is part of the themed collection: International Year of Crystallography Celebration: North America

 Please wait while we load your content...
Please wait while we load your content...