Structure and thermodynamics of a multimeric cavitand assembly†
Abstract
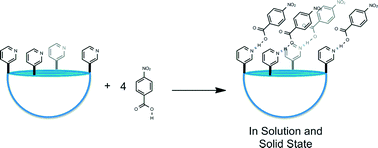
A set of COOH⋯N(pyridine) hydrogen bonds have been utilized in the targeted synthesis of a cavitand-based assembly. A cavitand appended with four pyridyl groups was synthesized from a Suzuki coupling of a tetrabromocavitand and 3-pyridyl boronic acid. The product resulting from the reaction between the tetra-pyridyl cavitand and 4-nitrobenzoic acid was investigated in solution using isothermal titration calorimetry (ITC) under equilibrium conditions, and in the solid state through single-crystal X-ray diffraction and infrared spectroscopy. The results show that the targeted pentameric hydrogen-bonded architecture was isolated in the solid-state, and the infrared spectroscopy confirm the presence of COOH⋯N(py) interactions. The same assembly, with a 1 : 4 cavitand : acid stoichiometry, is also present in solution in acetonitrile and the enthalpy of binding for this reaction is approximately −45 kJ mol−1.
- This article is part of the themed collection: Structural Macrocyclic Supramolecular Chemistry

 Please wait while we load your content...
Please wait while we load your content...