Crystal engineering of homochiral molecular organization of naproxen in cocrystals and their thermal phase transformation studies†
Abstract
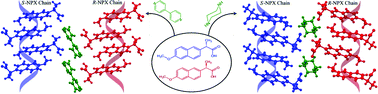
Since the racemic naproxen does not have the desired crystal structure essential to induce preferential enrichment, we used crystal engineering principle to produce the required homochiral R- and S-chains in the solid state. The cocrystal structures of racemic and S-naproxen (NPX) with bipyridine (BPY) and piperazine (PIZ) were determined, which consist of homochiral 1D naproxen chains that are associated by weak non-covalent interactions. Thermal studies of both racemic and S-naproxen–bipyridine cocrystals indicated a monotropic polymorphic transformation upon heating and the new crystalline phase was characterized by DSC, PXRD, hot-stage microscopy, and FT-IR spectroscopy.
- This article is part of the themed collection: Functional Co-crystals

 Please wait while we load your content...
Please wait while we load your content...