Synthesis and solid-state structures of a macrocyclic receptor based on the 2,6-bis(2-anilinoethynyl)pyridine scaffold†
Abstract
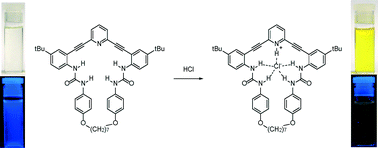
A fluorescent macrocyclic anion receptor based on the 2,6-bis(2-anilinoethynyl)pyridine scaffold has been synthesized to investigate the mechanism of fluorescence quenching in this class of compounds. X-ray crystallography reveals that the binding pocket of the receptor is a natural host to both H2O and HCl, accommodating either molecule in nearly identical environments. Our studies show that protonation, not collisional quenching, is responsible for the observed fluorescence quenching response.
- This article is part of the themed collection: Structural Macrocyclic Supramolecular Chemistry

 Please wait while we load your content...
Please wait while we load your content...