Cocrystallization with flufenamic acid: comparison of physicochemical properties of two pharmaceutical cocrystals†
Abstract
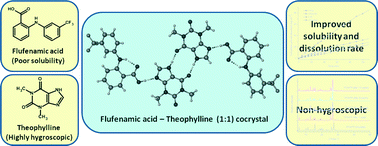
Three novel cocrystals of a non-steroidal anti-inflammatory drug, flufenamic acid (FFA) with theophylline (TP), 2-pyridone, and 4,4′-bipyridine were discovered in a cocrystal screening. All the cocrystals were thoroughly characterized and their crystal structures were determined. In the crystal structures, the FFA molecule interacts with the coformers via heterosynthons. The pharmaceutical relevance of the FFA·TP cocrystal prompted us to investigate its physicochemical properties and compare it with a reported FFA cocrystal with nicotinamide. In particular, properties such as stability, hygroscopicity, solubility, and dissolution rate were measured. FFA·TP was found to offer better solubility and dissolution rate; in addition, it also helps to circumvent the hygroscopic nature of TP. The fact that the FFA·TP cocrystal involves two active ingredients and shows better physicochemical properties than all other known cocrystals of FFA makes it a promising cocrystal for development of FFA formulations and combination drug products.
- This article is part of the themed collection: Functional Co-crystals

 Please wait while we load your content...
Please wait while we load your content...