The atomic origin of high catalytic activity of ZnO nanotetrapods for decomposition of ammonium perchlorate†
Abstract
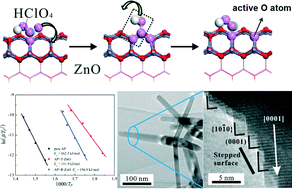
Distinct from the common well faceted ZnO nanorods (R-ZnO), ZnO nanotetrapods (T-ZnO) exhibited a remarkable catalytic activity for the thermal decomposition of ammonium perchlorate (AP): the activation energy at high temperature decomposition (HTD) was significantly decreased to 111.9 kJ mol−1, much lower than 162.5 kJ mol−1 for pure AP and 156.9 kJ mol−1 for AP with R-ZnO. This was attributed to more abundant atomic steps on the surface of T-ZnO than that of R-ZnO, as evidenced by HRTEM and density function theory (DFT) calculations. It was shown that the initiation step of perchloric acid (PA) decomposition happened much faster on stepped T-ZnO edges, resulting in the formation of active oxygen atoms from HClO4. The formed oxygen atoms would subsequently react with NH3 to produce HNO, N2O and NO species, thus leading to an obvious decrease in the activation energy of AP decomposition. The proposed catalytic mechanism was further corroborated by the TG-IR spectroscopy results. Our work can provide atomic insights into the catalytic decomposition of AP on ZnO nanostructures.

 Please wait while we load your content...
Please wait while we load your content...