Coverage-driven dissociation of azobenzene on Cu(111): a route towards defined surface functionalization†
Abstract
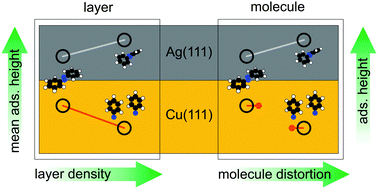
We investigate the surface-catalyzed dissociation of the archetypal molecular switch azobenzene on the Cu(111) surface. Based on X-ray photoelectron spectroscopy, normal incidence X-ray standing waves and density functional theory calculations a detailed picture of the coverage-induced formation of phenyl nitrene from azobenzene is presented. Furthermore, a comparison to the azobenzene/Ag(111) interface provides insight into the driving force behind the dissociation on Cu(111). The quantitative decay of azobenzene paves the way for the creation of a defect free, covalently bonded monolayer. Our work suggests a route of surface functionalization via suitable azobenzene derivatives and the on surface synthesis concept, allowing for the creation of complex immobilized molecular systems.


 Please wait while we load your content...
Please wait while we load your content...