Structurally simple complexes of CO2†
Abstract
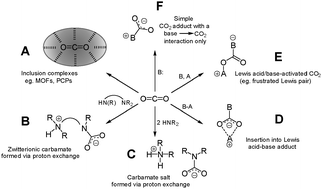
The ability to bind CO2 through the formation of low-energy, easily-broken, bonds could prove invaluable in a variety of chemical contexts. For example, weak bonds to CO2 would greatly decrease the cost of the energy-intensive sorbent-regeneration step common to most carbon capture technologies. Furthermore, exploration of this field could lead to the discovery of novel CO2 chemistry. Reduction of complexed carbon dioxide might generate chemical feedstocks for the preparation of value-added products, particularly transportation fuels or fuel precursors. Implementation on a large scale could help to drastically reduce CO2 concentrations in the atmosphere. However, literature examples of weakly bonded complexes of CO2 are relatively few and true coordination complexes to a ‘naked’ CO2 fragment are nearly unheard of. In this review article, a variety of complexes of CO2 featuring diverse binding modes and reactivity will be examined. Topics covered include: (A) inclusion complexes of CO2 in porous materials. (B) Zwitterionic carbamates produced from the reaction of CO2 with polyamines. (C) Carbamate salts produced from reaction of CO2 with two equivalents of an amine. (D) Insertion products of CO2 into acid-base adducts (e.g., metal complexes). (E) Lewis acid–base activated CO2, such as frustrated Lewis pair complexes. (F) Simple base–CO2 adducts, wherein the base–CO2 bond is the only interaction formed. Complexes in the last category are of particular interest, and include imidazol-2-carboxylates (N-heterocyclic carbene adducts of CO2) as well as a few other examples that lie outside NHC chemistry.

 Please wait while we load your content...
Please wait while we load your content...