A disulfide polymerized protein crystal†
Abstract
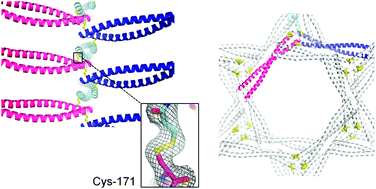
The vDED coiled coil domain from human BAP29 was crystallized in dimeric and tetrameric forms. For the dimer, a disulfide bond was unexpectedly found to bridge a crystal contact, resulting in complete cross-linking along the c-axis. This indicates that it is in principle possible to design spontaneously polymerizing protein crystals.

 Please wait while we load your content...
Please wait while we load your content...