Catalytic asymmetric Diels–Alder reactions involving aryl vinyl ketones†
Abstract
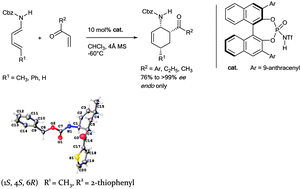
A catalytic asymmetric Diels–Alder reaction of an aryl vinyl ketone with 1,3-dienylcarbamate has been developed. Cyclohexenes bearing vicinal amino and aroyl groups in a cis-configuration were prepared in excellent ee (>99%) and endo (single diastereomer) selectivity. The absolute configuration of one DA product was unambiguously confirmed using XRD analysis. The transition state structure was proposed on the basis of DFT calculations.

 Please wait while we load your content...
Please wait while we load your content...