Redox-induced fluoride ligand dissociation stabilized by intramolecular hydrogen bonding†
Abstract
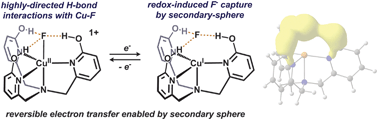
Chemical reduction of a tripodal Cu(II)–F complex containing pendent hydroxyl groups results in the partial dissociation of a F− ligand from Cu. The resulting Cu(I) complex is characterized as containing an outer sphere F− anion ‘captured’ by hydrogen bonds. The pendent hydroxyl groups were found to be crucial for reductive stability.
- This article is part of the themed collection: 2015 Emerging Investigators

 Please wait while we load your content...
Please wait while we load your content...