Mechanism of action of the uridyl peptide antibiotics: an unexpected link to a protein–protein interaction site in translocase MraY†
Abstract
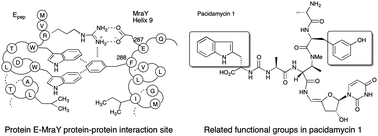
The pacidamycin and muraymycin uridyl peptide antibiotics show some structural resemblance to an Arg-Trp-x-x-Trp sequence motif for protein–protein interaction between bacteriophage ϕX174 protein E and E. coli translocase MraY. Members of the UPA class, and a synthetic uridine–peptide analogue, were found to show reduced levels of inhibition to F288L or E287A mutant MraY enzymes, implying that the UPAs interact at this extracellular site as part of the enzyme inhibition mechanism.

 Please wait while we load your content...
Please wait while we load your content...