The perils of rational design – unexpected irreversible elimination of fluoride from 3-fluoro-2-methylacyl-CoA esters catalysed by α-methylacyl-CoA racemase (AMACR; P504S)†
Abstract
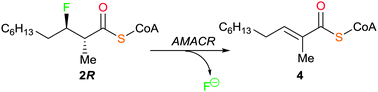
α-Methylacyl-CoA racemase (AMACR; P504S) catalyses ‘racemization’ of 2-methylacyl-CoAs, the activation of R-ibuprofen and is a promising cancer drug target. Human recombinant AMACR 1A catalyses elimination of 3-fluoro-2-methyldecanoyl-CoAs to give E-2-methyldec-2-enoyl-CoA and fluoride anion, a previously unknown reaction. ‘Racemization’ of 2-methyldec-3-enoyl-CoAs was also catalysed, without double bond migration.

 Please wait while we load your content...
Please wait while we load your content...