Synthesis of 1,2-amino alcohols via catalytic C–H amidation of sp3 methyl C–H bonds†
Abstract
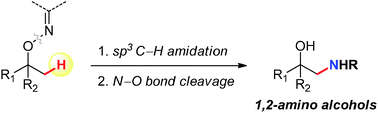
Herein a new synthetic route to 1,2-amino alcohols is presented by using C–H amidation of sp3 methyl C–H bonds as a key step. Readily available alcohols were employed as starting materials after converting them to removable ketoxime chelating groups. Iridium catalysts were found to be effective for the C–H amidation, and LAH reduction was then used to furnish β-amino alcohol products.

 Please wait while we load your content...
Please wait while we load your content...