Abstract
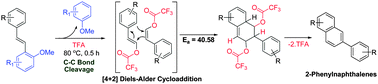
A new simple and efficient method for the synthesis of 2-phenylnaphthalenes from electron-rich 1-styryl-2-methoxybenzenes has been described. The reaction proceeds via TFA catalyzed C–C bond cleavage followed by intermolecular [4+2]-Diels–Alder cycloaddition of an in situ formed styrenyl trifluoroacetate intermediate. The quantum chemical calculations identified the transition state for the cycloaddition reaction and helped in tracing the reaction mechanism. The method has been efficiently utilized for synthesis of the phenanthrene skeleton and a naphthalene-based potent and selective ER-β agonist.

 Please wait while we load your content...
Please wait while we load your content...