Acid-catalyzed acylation reaction via C–C bond cleavage: a facile and mechanistically defined approach to synthesize 3-acylindoles†
Abstract
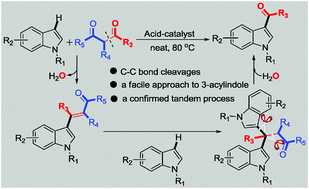
A facile acid-catalyzed acylation of indoles with 1,3-dione as an eco-friendly acylating agent was developed. This protocol combines C–C bond cleavage and heterocyclic C–H bond functionalization to form new C–C bonds. Based on the detailed mechanistic studies, a credible mechanistic pathway was proposed.

 Please wait while we load your content...
Please wait while we load your content...