Silver-catalyzed cyclization of 2-pyridyl alkynyl carbinols with isocyanides: divergent synthesis of indolizines and pyrroles†
Abstract
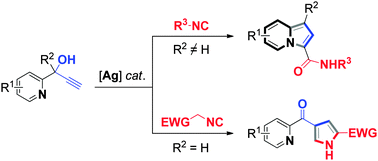
Divergent syntheses of indolizines and 2,4-disubstituted pyrroles by the silver-catalyzed cyclization of 2-pyridyl alkynyl carbinols with isocyanides are reported. These methods provide an effective route to highly functionalized indolizines and 2,4-disubstituted pyrroles in good to excellent yields. The 2,4-disubstituted pyrroles are synthesized by an unprecedented regioselective [3+2] cycloaddition of terminal alkynes with isocyanides.

 Please wait while we load your content...
Please wait while we load your content...